Emerging
|
Foundation
|
Core
|
Beyond Core
|
Accomplished
|
Can investigate and then determine which everyday metals easily corrode
|
Can observe and describe single displacement reactions involving metals
|
Can observe and describe reasons for the reactivity series in metals
|
Can describe why certain periodic table group elements are more reactivity than other groups and predict the reactivity series down a column
|
Can describe different crystal structures in metals and how they affect its physical properties
|
Week 5/6 TASKSHEET
LEO GER
Loss of Electrons = Oxidation
eg. Al(s) --> Al3+ (aq) + 3 e-
Gain of Electrons = Reduction
eg. O2 (g) + 2e- ---> O2-
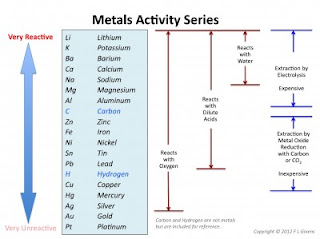
DISPLACEMENT REACTIONS (precipitation)
The difference between the SINGLE and DOUBLE displacement reactions is shown below in this image.......
A, B, C & D all represent atoms of elements.....
SINGLE displacement reactions are the first image - A&B are chemically joined to form a compound and C is all on its own. When they react together, B swaps to become chemically joined to C to form a compound and A ends up all on its own.
Example: HCl + Zn --> H2 + ZnCl
DOUBLE displacement reactions are what we were doing last week - where both compounds swap partners.
Example: CaBr + KOH --> CaOH + KBr


No comments:
Post a Comment